

Oxidation and Reduction in Chemical Cells.
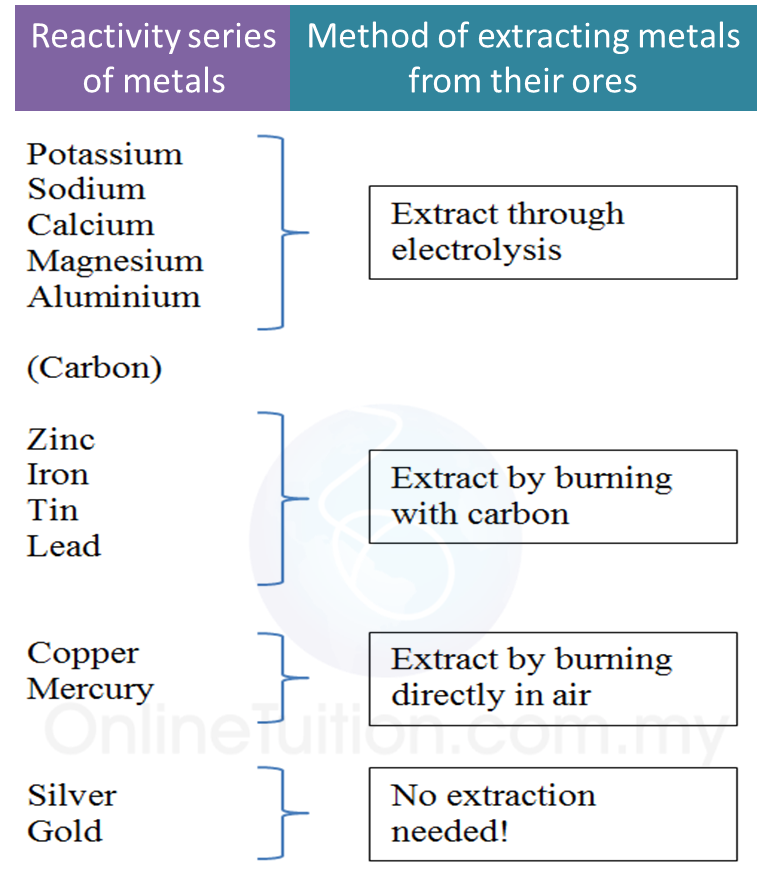
Oxidation and Reduction in Electrolytic Cells.Application of the reactivity series of metals in the extraction of metals.Redox Reactions by Transfer of Electrons at a Distance.Displacement of Halogen From Halide Solution.Redox reaction in the displacement of metals from its salt solution.Changing of iron(II) ions to iron(III) ions and vice versa.This series of metals is quite similar to the electrochemical series because the reactivity of a metal with oxygen is closely linked to its ability to lose electrons. The reactivity series of metals towards oxygen is a list of metals according to their reactivity with oxygen.Hence, by observing how vigorously the metals react with oxygen, we can arrange the metals according to their reactivity towards the oxygen.The more reactive a metal is towards oxygen, the more vigorously it burns in oxygen.(c) Thus, metal acts as the reducing agent while oxygen acts as the oxidising agent in the formation of metal oxide. Its oxidation number decreases from 0 to -2. (b) Oxygen undergoes reduction to form oxide ions. Its oxidation number increases from zero to a positive value. (a) Metal undergoes oxidation to form positive ions. The formation of metal oxide is a redox reaction. One of the common compounds formed by metals is metal oxide.



 0 kommentar(er)
0 kommentar(er)
